Iron in water can exist in different states, namely :
- Divalent iron – this can be any compounds involving iron in divalent form. For example, in open sources it can be humic acid iron compounds, organic-mineral complexes, iron sulfate FeSO4, iron sulfide FeS, suspensions of iron hydroxide Fe(OH)2. In underground springs, iron bicarbonate Fe(HCO3)2.
- Trivalent iron is most often iron hydroxide Fe(OH)3. Many divalent iron compounds are soluble in water. They are not retained by mechanical filters and do not affect the color of the water. Often the water from a well (say, 30 meters deep) at the first moment after the selection is perfectly clear and has no signs of turbidity, but after a few minutes to a few hours, the water is turbid. After a few hours, the turbidity settles, forming a brown friable sediment. Trivalent iron hydroxide, which is the most common of all trivalent iron compounds, is insoluble in water.
Concentrations of iron in groundwater sources can range from 0.5-50mg/L. Starting with a concentration of 1.0-1.5 mg/l, the water has an unpleasant metallic taste. And when the water is more than 0,3mg/l, the water leaves stains on the laundry and plumbing.
SanPin 2.2.4-171-10 regulates the iron content in drinking water not more than 0.2mg/l.
Methods for Deferrization of Water
Of the methods of deferrization can be distinguished two main directions:
- Removal of iron from water in the undissolved form.
- The removal of iron from water in dissolved form.
- Non-dissolved iron removal is limited to mechanical filtration of water by means of sand filters or other filters capable of retaining particles up to 1 micron.
- The removal of iron in dissolved form is a complex technological process, which consists of several stages and is regulated differently for different types and concentrations of dissolved iron in water. However, in any case, the essence of the process is to convert iron from dissolved form to insoluble form with subsequent filtration on mechanical filters.
There are several ways to deferrize water:
- Reagentless non-pressure aeration followed by filtration.
- Reagent-free pressure aeration followed by filtration.
- Reagent-free pressure aeration followed by filtration.
- Reagent-free pressure aeration followed by filtration.
- Filtration with the use of catalytic loads.
The first two methods are used under the following conditions:
- Iron content not more than 10mg/l.
- Ph not less than 6,8.
- Hydrogen sulfide content not more than 2mg/l.
- The content of ammonium salts no more than 1mg/l.
- Sulfides content 0,2 mg/l or less.
- Total alkalinity not more than 28 mmol/l.
- Permanganate acidity not more than 3 mgO/l.
The essence of the first method is that the water is sprayed in an unpressurized container and falls through a layer of air to the bottom of the container by means of a smothering unit 1. From the bottom of the tank water is sucked by the pump P1 and fed to the filter of mechanical cleaning of the bulk type F1. The volume of the tank is selected in such a way that the tank is filled no more than 30%, and the contact time is not less than 30 minutes. Part of the water from the pressure line of the filter is returned back to the tank through the barbater 2, located in the lower part of the tank, it is necessary to prevent the formation of a thick layer of iron hydroxide sludge at the bottom of the tank.
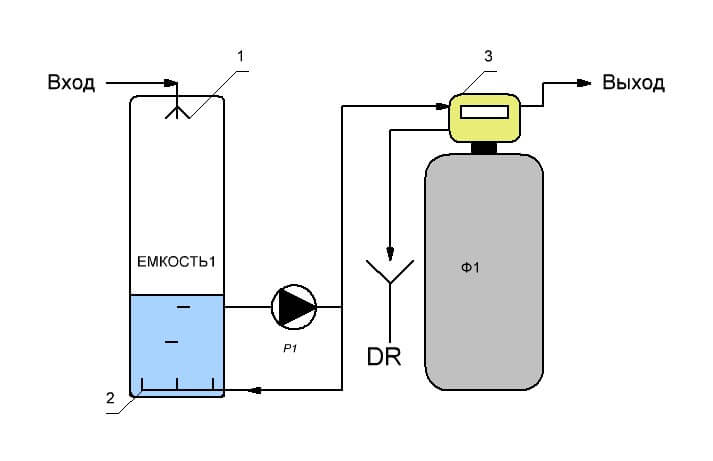
The iron hydroxide is filtered from the water on the filter loading F1 and is periodically removed from the loading by backwashing, carried out by the automatic flow distribution device 3.
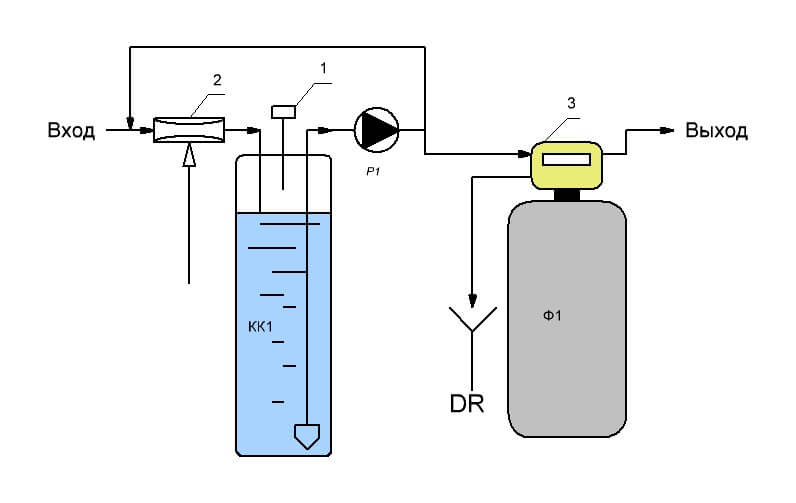
The second method differs from the first in that air is introduced into the water by means of an ejector 2. The air-water mixture is fed into the contact tank KK1, to provide oxidation time and air removal from the mixture. After air removal by deaerator valve 1, the water is subjected to mechanical filtration similar to the first method.
The third method – reagent-free treatment with subsequent filtration is used if the iron in water is contained in the form of iron sulfate FeSO4. In this case, the aeration method is unacceptable, because in the process of hydrolysis of the dissolved salt, Ph falls below 6.8 and the process virtually stops. To remove the iron sulfate, liming of water with subsequent filtration of the reaction products, namely iron hydroxide and gypsum, is used.
FeSO4+Ca(OH)2 = Fe(ON)2+CaSO4
Industrial deferrization of water: This technique is often used in industry to treat surface water for iron removal.
Reagent pressure treatment is used when the use of reagentless aeration is not possible for reasons that do not allow the use of reagentless aeration. Reagent water treatment resembles the second method, only instead of air, a chemical that oxidizes iron compounds to trivalent hydroxide is introduced into the water.
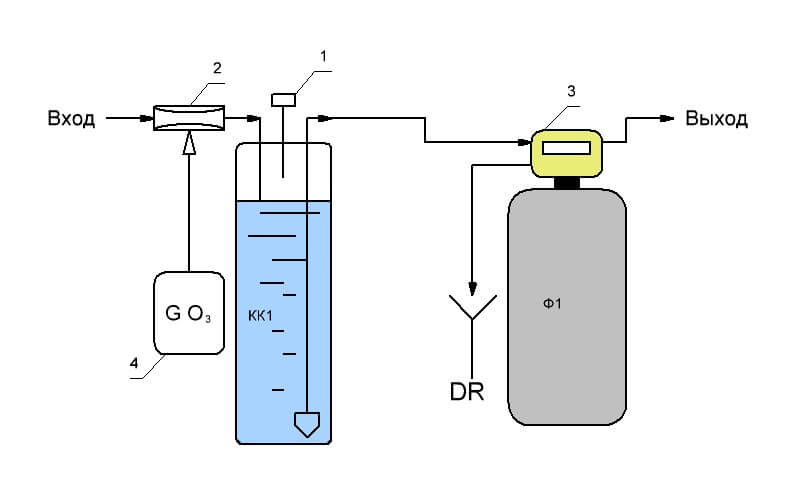
As an oxidizing agent, ozone is often used, produced by a special ozone generator 4, which is injected into the water by ejector 2. Then there is a process of separation of ozone in the contact column KK1, valve 1 and further processing on a mechanical filter. Potassium permanganate, sodium hypochlorite, chlorine and its derivatives can also be used as an oxidizer. The difference consists in different dosing devices and devices for controlling the concentration of reagent in water.
The last method – filtration with the use of catalytic loads is fundamentally different from all previous methods. Catalytic loads are natural materials containing manganese dioxide or materials into which manganese dioxide is introduced through treatment. Such loads include Pyrolox, Aquamandix, “black sand”, sulpho coal, MZF, Birm, Green Sand, MTM.
The mechanism of action is based on the fact that manganese dioxide is able to change the valence state quite easily. Oxidized on the surface of the fill pellets and trapped iron, is subsequently washed away by the backwash flow. The reacted loading material is either regenerated or replaced with new material.
Disadvantages of this method:
- Backfills are ineffective against organic iron.
- The pellets are blocked by organics and lose their properties.
- Often the limit of application is the iron content of more than 15mg/l.
- Increased manganese content in water reduces the effectiveness of deferrization.
Mixed methods involving, for example, pressure aeration and filtration on catalytic loading are often used. In this case, a mixture consisting of 20% of catalyst and 80% of sand is poured into the sand filter. The catalyst de-oxidizes the iron, which for some reason was not oxidized in the contact column.
In any case, as for catalyst loads, it is accepted filtration speed not more than 10m/h, and backwashing speed 25-30m/h.
In this article we gave answers to the questions: “How to remove iron from water”. Deferrization of water at the cottage, at home – the technology is identical.
