Modern reverse osmosis systems are widely used for desalination and water purification in industry and in the home. To understand the capabilities of installations that use the principle of reverse osmosis, you need to understand in general terms what the reverse osmosis process is.
The principle of reverse osmosis
Osmosis is
Osmosis (from the Greek for “push, pressure”) is the process of diffusion of a solvent from a less concentrated solution into a more concentrated solution.
The process of osmosis occurs on semipermeable membranes, in nature these are cell membranes.
A semipermeable membrane is a membrane that has a sufficiently high permeability not for all, but only for some substances, in particular, for a solvent.
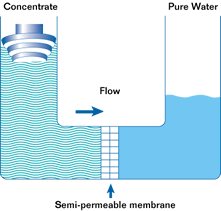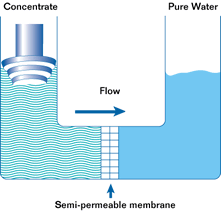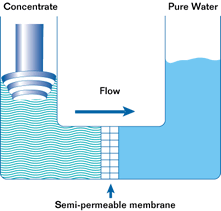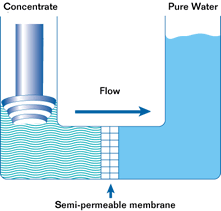
If such a membrane is used to divide a container with a less concentrated solution in one part and a more concentrated solution in the other, water from the solution with a lower concentration will pass through the membrane into the solution with a higher concentration. This will increase the pressure (level) in the container with the higher concentration. This process is not endless. The process of osmosis will continue until the pressure in the container with the more concentrated solution increases to a point where the diffusion process stops. This pressure is called the osmotic pressure.
Osmotic pressure is the pressure that must be created in a container with a more concentrated solution in order to stop the osmosis process.
The most important property of osmosis is that when the pressure in a container with a more concentrated solution exceeds the osmotic pressure, the osmosis process is reversed and reverse osmosis begins.
Reverse osmosis is the passage of a solvent through a semipermeable membrane from a more concentrated to a less concentrated solution due to the influence of a pressure exceeding the difference in osmotic pressures of both solutions. In this case, the membrane allows the solvent to pass through, but does not allow some of the substances dissolved in it to pass through.
A semi-permeable reverse osmosis membrane does not retain all substances dissolved in water. It retains only high molecular weight pollutants, such as salts, viruses, bacteria, and colloids. At the same time, the membrane allows low-molecular weight substances, such as oxygen, chlorine, and carbon dioxide to pass through. Low molecular weight substances must be removed before the water enters the reverse osmosis plant, as many of them adversely affect the operation of the plant and the life of the membranes.
Installation of reverse osmosis
Semi-permeable membranes are produced by the industry in the form of films, which are rolled into rolls for compactness. In such rolls, in addition to the membrane itself, there are all kinds of elements to separate the flows and create conditions for the normal operation of the membrane. A semi-permeable membrane rolled up and installed in a special sealed pipe holder is called a membrane module.
In a reverse osmosis plant can be from one to hundreds of such modules. In the membrane module, the source water is divided into two streams. Pure water is called permeate. Water with a high concentration of contaminants is called concentrate. Permeate is used for its intended purpose, and the concentrate is discharged into the sewer or overboard.
An industrial reverse osmosis plant is a complex system whose task is to ensure the normal operation of the membrane modules. For example, to provide the required pressure in the membrane module from 8 to 80 MPa, depending on the purpose of the installation, to ensure optimal water flow through the module to prevent clogging of the membranes, to ensure periodic washing of the membranes with clean water and chemicals to remove contaminants.
In some cases, industrial plants are equipped with dosing systems for inhibitors of deposits – antiscalants, to prevent salt deposits on membranes in the event of a high concentration of salts in the source water. Control systems for industrial plants can be either fully automatic or semi-automatic. The plants can be equipped with devices for rapid analysis of the main parameters of water.
Reverse osmosis for home is a combined water treatment system. It usually consists of three pre-filters: a 5 micron mechanical filter to retain mechanical impurities, a carbon filter to purify water from chlorine and some organic compounds, and a 1 micron mechanical filter to remove small mechanical contaminants and fragments of the carbon filter that can be washed out of it by the water flow.
This is followed by the reverse osmosis membrane itself, the concentrate is washed off into the sewer, and the permeate is fed into a storage tank with a capacity of usually 9-10 liters. The system is equipped with a shut-off valve that automatically shuts down the unit after the storage tank is full.
Sometimes such a system is equipped with a post-carbon filter for the removal of low molecular weight substances, a mineralizer for saturating the water with useful minerals and a bioceramic activator for partial structuring of water. Such a system can also be equipped with an electric pump with automation necessary for operation in conditions of reduced source water pressure.
